MigraGel
Bicontinuous aqueous two-phase systems based on GelMA and dextran for tailored porous hydrogels in 3D tissue engineering
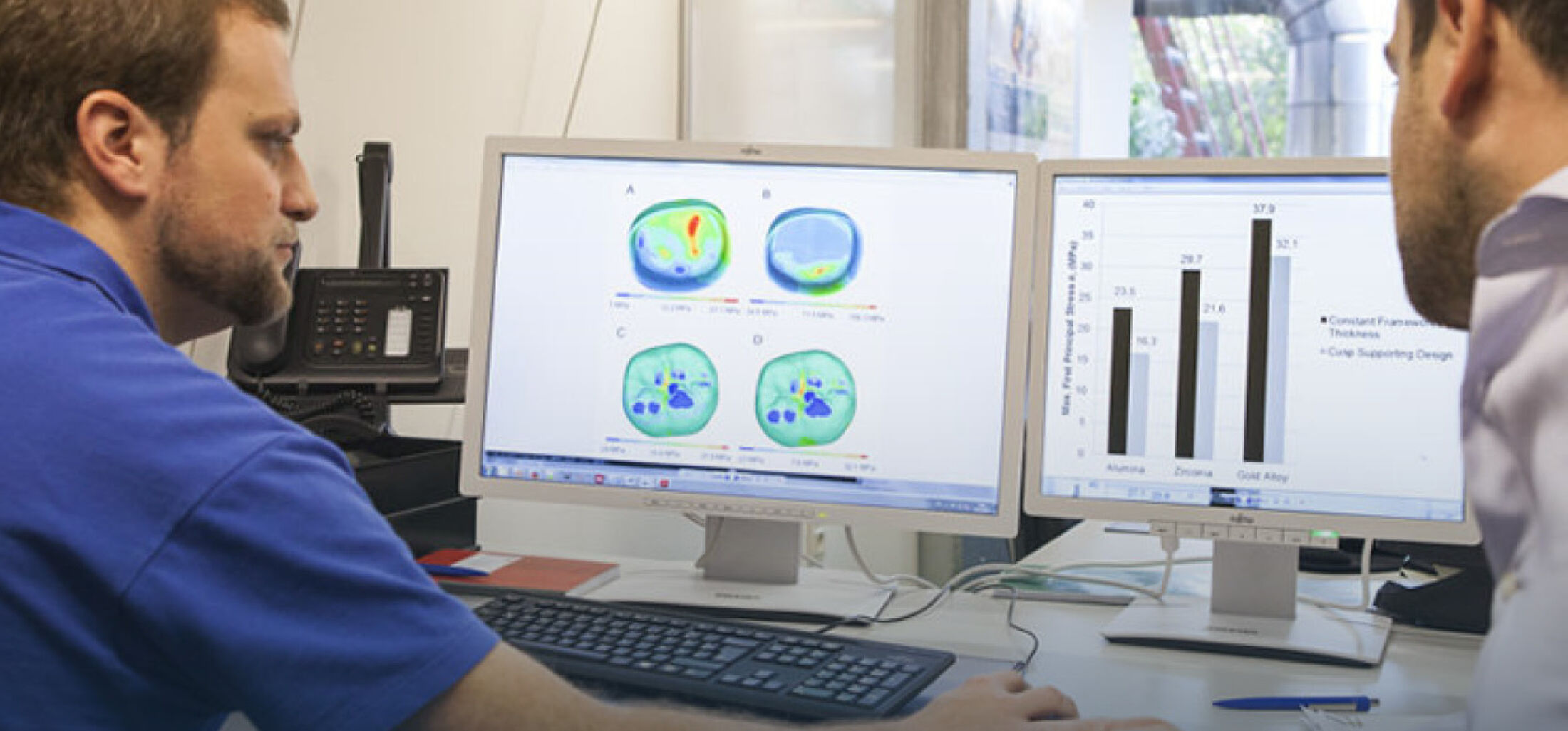
Hydrogels are polymeric materials able to absorb and retain a large amount of water in their three-dimensional (3D) structure, making them ideal material candidates as substitutes for the natural extracellular matrix (ECM) in tissue engineering applications. Besides this trait, many hydrogels can be tuned regarding their mechanical behavior and their biocompatibility. This way, these hydrogels can be adjusted accordingly to mimic the specific ECM requirements demanded by distinct types of cells. Of such characteristics, and of utmost importance, is the material’s microstructure and porosity, which plays a significant role in augmenting or diminishing the cells’ abilities to proliferate, spread, migrate, and differentiate.
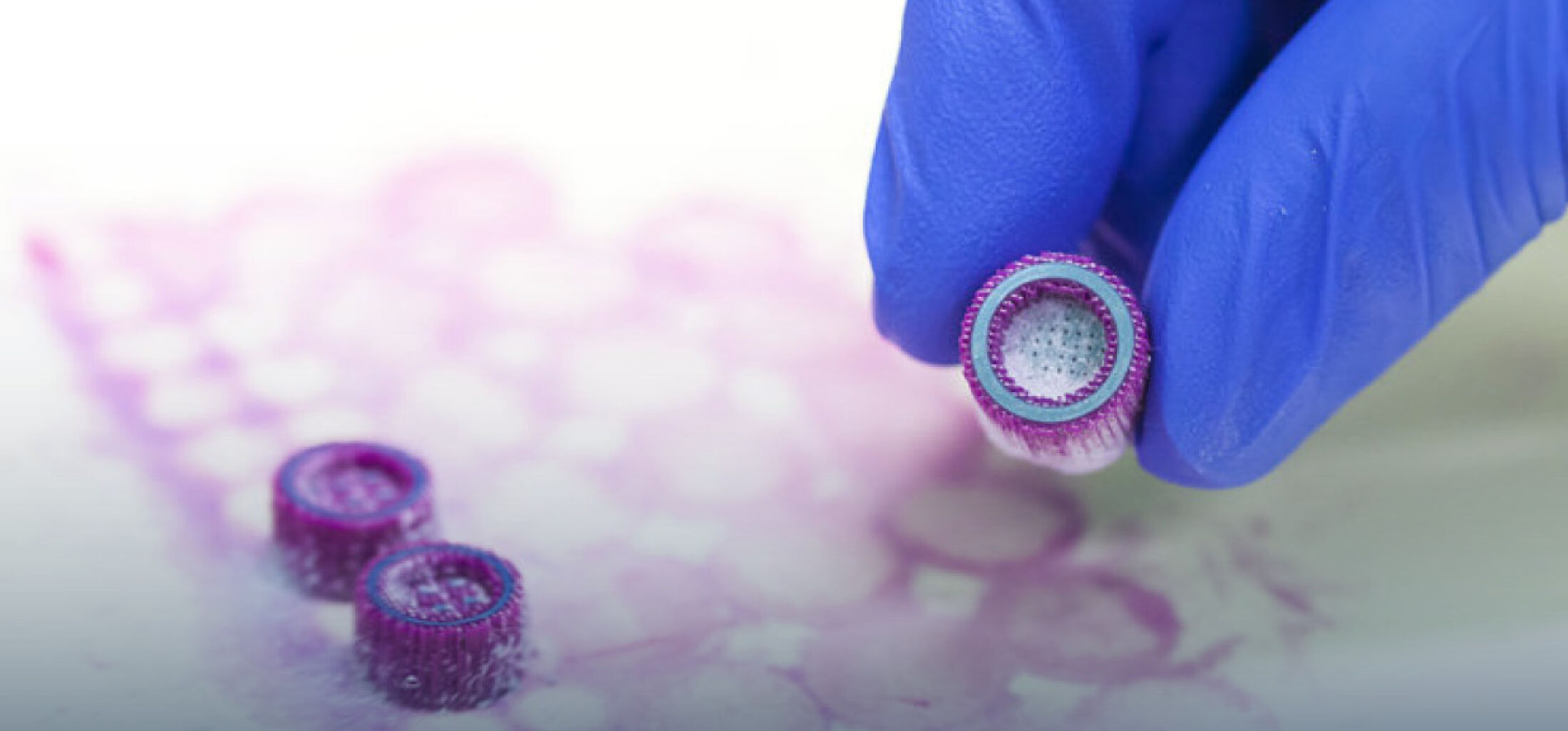
The project conducted together with the group of Prof. Richtering (RWTH Aachen University) and funded by the German Research Foundation (DFG) aims to synthesize and characterize a novel hydrogel using a bicontinuous aqueous two-phase system (ATPS) based on gelatin-methacryloyl (GelMa) and dextran. Proof-of-concept experiments have shown that by fine-tuning the phase separation mechanism and kinetics, it is possible to attain homogeneous disconnected-porous and bicontinuous interconnected-porous hydrogels by crosslinking the GelMa phase and washing out the dextran phase. The bicontinuous microstructure showed promising results (Figure 1). The material is stable after bioprinting and can maintain cell viability and morphology of different cells for seven days.
Thus, to better understand and the novel hydrogels and to optimize their microstructure, it is important to understand the underlying physico-chemical principles that govern the phase separation. With this information, the hydrogel will be processed and its mechanical properties characterized. Its applicability for 3D bioprinting will be evaluated using the microextrusion and inkjet (drop-on-demand) techniques and the effect these processes have on the microstructure and the mechanical characteristics of the hydrogel will be examined. Furthermore, with the use of diverse cell types, the casted and printed hydrogels’ affinity in promoting cellular growth and viability will be investigated. This will give an insight into its applicability for regenerative medicine, dental studies, and cancer research. The biofunctionality of the hydrogel will then be investigated by examining the behavior of cytokines (partitioning and release) in GelMa/dextran ATPS hydrogels. Finally, it will be examined the influence that embedded cells in the ATPS solution have on the bioprinting and phase separations processes as a function of the resulting hydrogel’s microstructure. Likewise, the effect the bioprinting process has on the cells’ viability, proliferation and ECM adhesion will be analyzed and compared to those in casted hydrogels.