NeWTranslation – Sub-Project CeramActive
Osteoarthritis is a common chronic disease of the elderly. Arthritis is characterized, among other things, by the progressive loss of articular cartilage, which increasingly leads to pain and stiffness in the affected joints. For this reason, about 400.000 hip and knee endoprostheses are implanted annually in Germany alone. Additionally surgical revisions of the implants due to aseptic loosening are increasing.
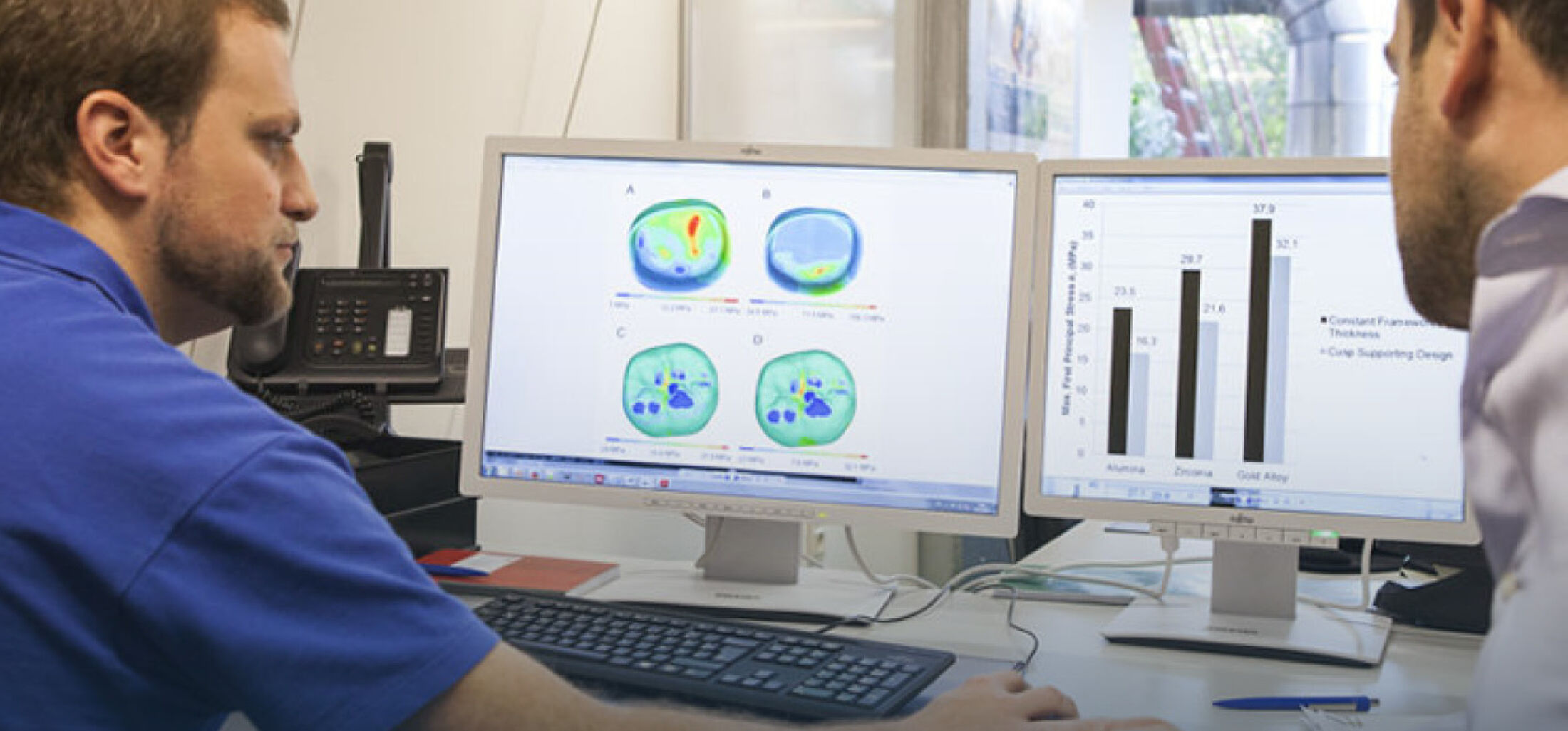
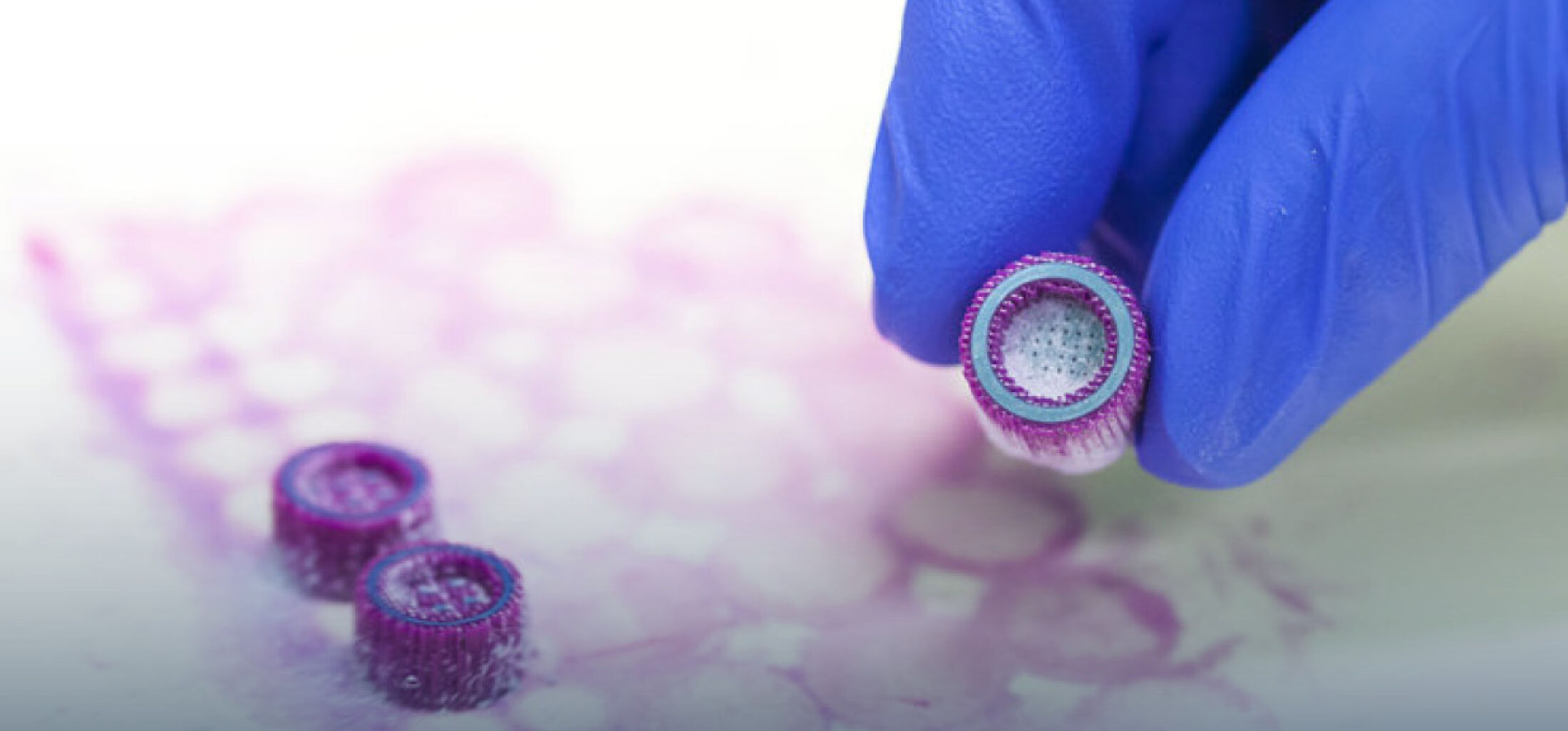
In basic scientific preliminary research we were already able to show that specific biomolecules can be immobilized at the surface of high‑performance ceramics in a hydrolytical-stable manner by a multilayer organochemical process (Figure 1). In vitro studies have confirmed that such a biofunctionalization can lead to an enhanced osseointegration of the bioinert ceramic implant surface. This could clinically contribute to less aseptic loosening and thus to a significantly longer service life of an all-ceramics implant. This technique still has to go through an extensive approval process before it can actually be used as a medical product (Figure 2). However, many companies do not have the financial resources to make high upfront investments for the required conformity assessment and approval. Therefore, innovations such as this coating technology are often not translated into clinical application. In order to improve translation from university research into an approved medical product the CeramActive sub-project is funded by the German Federal Ministry of Education and Research (BMBF), as part of the NeWTranslation joint project. The aim is to prepare the research results already achieved and validated by basic science for a first clinical application in humans.

To this, the requirements for the approval of the coating technology in accordance with the Medical Device Regulation (MDR) are being developed in close coordination with internal project partners. In addition, the coating technology will be optimized in terms of hydrolytic stability and examined particularly with regard to sterilizability. Finally, the coating process is to be transferred to a GMP-compliant working environment (Good Manufacturing Practice). The project, which is based on the regulations of the MDR, is intended to subsequently transfer the further developed technology into concrete applications for patient care together with medical devices companies.