Koch Group
Netzwerkeigenschaften und Mikrostruktur des menschlichen Gehirns
Das menschliche Gehirn muss ständig die kontinuierliche, elektrische Aktivität anpassen, welche die Grundlage von Informationsverarbeitung und der Gedächtnisbildung darstellt. Eine Fehlregulation dieses Gleichgewichts kann die neuronalen Netze in einen übererregten Zustand versetzen, was zu sogenannten epileptischen Anfällen führt, die eine Störung der betroffenen Hirnregionen verursacht. Die Ursache von epileptischer Hirnaktivität kann sehr unterschiedlich sein und die Mechanismen, die an der Entstehung und Entwicklung einer epileptischen Störung beteiligt sind, sind sehr komplex. Das Ziel unserer Arbeitsgruppe ist es, die Erregbarkeit kortikaler Verschaltungen und die Plastizität menschlicher Neurone und neuronaler Netzwerke zu untersuchen, auch inwieweit sie an der Epileptogenese beteiligt sind. Die Identifizierung potenzieller molekularer und zellulärer Signalwege, die an der Regulierung der Erregbarkeit der kortikalen Netzwerke beteiligt sind, könnte neue Ansatzpunkte für eine frühzeitige Intervention bei der Behandlung von Epilepsie aufdecken.
Dr. Henner Koch
hkochukaachende
Address
Dept. of Neurology, Section Epileptology
University of Aachen
Pauwelsstrasse 30
52074 Aachen, Germany
Tel. +49-(0)241- 80-80576
Team

Dr. Henner Koch
hkochukaachende
Address
Dept. of Neurology, Section Epileptology
University of Aachen
Pauwelsstrasse 30
52074 Aachen, Germany
Tel. +49-(0)241-80-80576

Aniella Bak
abakukaachende
Address
Dept. of Neurology, Section Epileptology
University of Aachen
Pauwelsstrasse 30
52074 Aachen, Germany
Tel. +49-(0)241 80-38786

Sebastian Maruri
smaruripazmiukaachende
Address
Dept. of Neurology, Section Epileptology
University of Aachen
Pauwelsstrasse 30
52074 Aachen, Germany
Tel. +49-(0)241-80675

Birgit Gittel (lead technician)
bgittelukaachende
Address
Dept. of Neurology, Section Epileptology
University of Aachen
Pauwelsstrasse 30
52074 Aachen, Germany
Tel. +49-(0)241-80-38611

Sabrina Peter, Bsc.
sapeterukaachende
Address
Dept. of Neurology, Section Epileptology
University of Aachen
Pauwelsstrasse 30
52074 Aachen, Germany

Katharina Schmied
kschmiedukaachende
Address
Dept. of Neurology, Section Epileptology
University of Aachen
Pauwelsstrasse 30
52074 Aachen, Germany

Kerstin Schünemann
kschuenemannukaachende
Address
Dept. of Neurology, Section Epileptology
University of Aachen
Pauwelsstrasse 30
52074 Aachen, Germany
Research projects and techniques
Aging and Neurodegeneration in a Human Brain Tissue Model
- Studying and manipulating the mature human brain at a cellular level has historically been challenging. Recent success involves long-term culturing of surgically resected human brain tissue while preserving its mature structure and neuronal activity.
- Recent research highlights the critical role of glia cells in neurodegenerative diseases.
- The project combines expertise in brain slice cultures and immunology to create a novel tool for exploring neurodegenerative disease dynamics and molecular mechanisms.
- This innovative system has the potential to bridge the gap between animal and human studies, serving as a primary tool for investigating neurodegenerative disease processes and testing new therapeutics.
- https://molecularneurodegeneration.biomedcentral.com/articles/10.1186/s13024-021-00471-2
- https://chanzuckerberg.com/science/programs-resources/neurodegeneration-challenge/projects/
- bridging-the-translational-gap-a-novel-adult-human-brain-tissue-system/
- Human organotypic brain slice cultures: a detailed and improved protocol for preparation and long-term maintenance - PubMed (nih.gov)
- Paper of the Quarter (PoQ) (3r-netzwerk.nrw)
Epilepsy and the respiratory control of breathing – Implication for SUDEP
- SUDEP is the leading epilepsy-related cause of death, affecting all ages and epilepsy severities. While ASMs control seizures for many patients, they don't cure epilepsy and can have severe side effects.
- Roughly 30% of ASM-resistant epilepsy patients face ongoing seizures and higher SUDEP risk, necessitating safer treatments.
- Targeting new molecular anti-seizure treatments may yield novel drugs and early diagnosis biomarkers.
- Cardiorespiratory dysfunction post-seizures may be SUDEP's common mechanism. Brainstem involvement due to epilepsy activity can cause SUDEP. Mouse models and advanced tech allow precise brain network investigations.
- A critical need persists for understanding epilepsy and SUDEP mechanisms. Mouse models and precise methods are vital for translating findings into preventive and therapeutic strategies.
- Promising therapies often fail to transition from preclinical models to human treatments.
- New therapy options are primarily developed and tested in non-human systems.
- Utilize human ex vivo brain systems to bridge the gap between preclinical and clinical research in epilepsy.
- Characterize and optimize viral vectors for gene therapy in human brain tissue, apply viral vector-mediated CRISPR techniques to model epilepsy causes in human samples, and use these insights to enhance promoter-driven gene therapy approaches for epilepsy. The ultimate goal is to improve treatment approaches for patients with epilepsy by gaining more accurate insights into disease mechanisms within a human context.
https://elifesciences.org/articles/48417
https://www.nature.com/articles/s41598-017-12527-9
https://www.biorxiv.org/content/10.1101/2023.10.10.561508v1
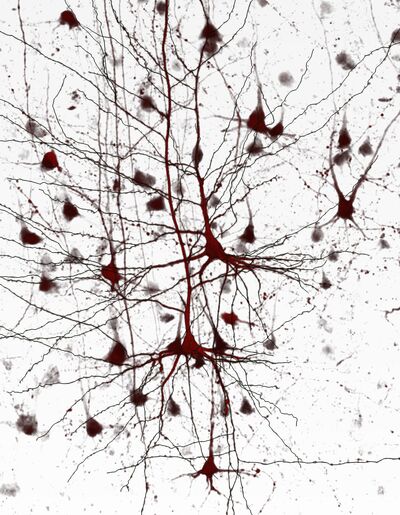
- Epilepsy involves synchronized depolarizations of neuron groups leading to seizures, with various causes. The electrophysiological and micro-anatomical aspects of seizures are poorly understood.
- To investigate epileptic discharges at a micro-network level we utilize innovative culturing methods for human brain slices and employ micro-electrode arrays (MEA) for spatio-temporal electrophysiological recordings.
- MEA recordings are instrumental in studying neuronal activity in human neocortical and hippocampal slices, providing insights into synchronized network activities. This approach aids in better understanding neuronal network dynamics and simulating physiological and disease-related brain processes.

Publications
10 Selected publications:
Koch H*, Zanella S, Elsen GE, Smith L, Doi A, Garcia AJ, Wei AD, Xun R, Kirsch S, Gomez CM, Hevner RF, Ramirez JM. 2013. Stable respiratory activity requires both P/Q-type and N-type voltage-gated calcium channels. J Neurosci 33:3633–3645. doi:10.1523/JNEUROSCI.6390-11.2013
Koch H, Caughie C, Elsen FP, Doi A, Garcia AJ, Zanella S, Ramirez JM. 2015b. Prostaglandin E2 differentially modulates the central control of eupnoea, sighs and gasping in mice. J Physiol 593:305–319. doi:10.1113/jphysiol.2014.279794
Koch H*, Huh S-E*, Elsen FP, Carroll MS, Hodge RD, Bedogni F, Turner MS, Hevner RF, Ramirez J-M. 2010. Prostaglandin E2-induced synaptic plasticity in neocortical networks of organotypic slice cultures. J Neurosci 30. doi:10.1523/JNEUROSCI.4665-09.2010
Koch H, Niturad CE, Theiss S, Bien CG, Elger C, Wandinger KP, Vincent A, Malter M, Körtvelyessy P, Lerche H, Dihné M. 2019b. In vitro neuronal network activity as a new functional diagnostic system to detect effects of Cerebrospinal fluid from autoimmune encephalitis patients. Sci Rep 9:1–8. doi:10.1038/s41598-019-41849-z
Koch H, Weber YG. 2019b. The glucose transporter type 1 (Glut1) syndromes. Epilepsy Behav 91:90–93. doi:10.1016/j.yebeh.2018.06.010
Schwarz N, Hedrich UBS, Schwarz H, Harshad PA, Dammeier N, Auffenberg E, Bedogni F, Honegger JB, Lerche H, Wuttke T V., Koch H*. 2017. Human Cerebrospinal fluid promotes long-term neuronal viability and network function in human neocortical organotypic brain slice cultures. Sci Rep 7:1–12. doi:10.1038/s41598-017-12527-9
Schwarz N, Uysal B, Welzer M, Bahr JC, Layer N, Löffler H, Stanaitis K, Harshad PA, Weber YG, Hedrich UBS, Honegger JB, Skodras A, Becker AJ, Wuttke T V.*, Koch H*. 2019. Long-term adult human brain slice cultures as a model system to study human CNS circuitry and disease. Elife 8. doi:10.7554/eLife.48417
Layer N, Brandes J, Lührs PJ, Wuttke T V., Koch H. 2021. The effect of lamotrigine and other antiepileptic drugs on respiratory rhythm generation in the pre-Bötzinger complex. Epilepsia 62:2790–2803. doi:10.1111/epi.17066
Kruszynski S, Stanaitis K, Brandes J, Poets CF, Koch H. 2019a. Doxapram stimulates respiratory activity through distinct activation of neurons in the nucleus hypoglossus and the pre-bötzinger complex. J Neurophysiol 121:1102–1110. doi:10.1152/jn.00304.2018
Wickham J, Corna A, Schwarz N, Uysal B, Layer N, Honegger JB, Wuttke T V., Koch H*, Zeck G*. 2020. Human Cerebrospinal Fluid Induces Neuronal Excitability Changes in Resected Human Neocortical and Hippocampal Brain Slices. Front Neurosci 14. doi:10.3389/fnins.2020.00283
Further publications
Alhaskir M, Bauer J, Linke F, Schriewer E, Weber Y, Wolking S, Röhrig R, Rothermel M, Koch H, Kutafina E. 2023a. Spectral Fusion of Heartbeat and Accelerometer Data for Estimation of Breathing Rate in Wearable Patches. Stud Health Technol Inform 302:1025–1026. doi:10.3233/SHTI230336
Bak A, Koch H, Van Loo KMJ, Schmied K, Gittel B, Weber Y, Schwarz N, Tauber SC, Wuttke T V, Delev D. 2023. Long-term human organotypic brain slice cultures: a detailed protocol to provide a comprehensive framework for single-neuron and neuronal network investigations. bioRxiv 2023.10.10.561508. doi:10.1101/2023.10.10.561508
Barth M, Bacioglu M, Schwarz N, Novotny R, Brandes J, Welzer M, Mazzitelli S, Häsler LM, Schweighauser M, Wuttke T V., Kronenberg-Versteeg D, Fog K, Ambjørn M, Alik A, Melki R, Kahle PJ, Shimshek DR, Koch H, Jucker M, Tanriöver G. 2021. Microglial inclusions and neurofilament light chain release follow neuronal α-synuclein lesions in long-term brain slice cultures. Mol Neurodegener 16:54. doi:10.1186/s13024-021-00471-2
Bauer J, Devinsky O, Rothermel M, Koch H. 2023a. Autonomic dysfunction in epilepsy mouse models with implications for SUDEP research. Front Neurol. doi:10.3389/fneur.2022.1040648
Czolk R, Schwarz N, Koch H, Schötterl S, Wuttke T V., Holm PS, Huber SM, Naumann U. 2019a. Irradiation enhances the therapeutic effect of the oncolytic adenovirus XVir-N-31 in brain tumor initiating cells. Int J Mol Med 44:1484–1494. doi:10.3892/ijmm.2019.4296
Dubey M, Brouwers E, Hamilton EMC, Stiedl O, Bugiani M, Koch H, Kole MHP, Boschert U, Wykes RC, Mansvelder HD, van der Knaap MS, Min R. 2018b. Seizures and disturbed brain potassium dynamics in the leukodystrophy megalencephalic leukoencephalopathy with subcortical cysts. Ann Neurol 83:636–649. doi:10.1002/ana.25190
Fischer FP, Karge RA, Weber YG, Koch H, Wolking S, Voigt A. 2023. Drosophila melanogaster as a versatile model organism to study genetic epilepsies: An overview. Front Mol Neurosci 16:1116000. doi:10.3389/fnmol.2023.1116000
Frese H, Kauth A, Koch H, Ort J, Ingebrandt S. 2023. Fabrication and characterization of flexible microelectrode arrays for the long-term recording of mammalian brain slices. Curr Dir Biomed Eng 9:375–378. doi:10.1515/cdbme-2023-1094
Garcia AJ, Zanella S, Koch H, Doi A, Ramirez JM. 2011. Networks within networks. The neuronal control of breathing. Prog Brain Res 188:31–50. doi:10.1016/B978-0-444-53825-3.00008-5
Hedrich UBS, Koch H, Becker A, Lerche H. 2019. Epileptogenesis and consequences for treatment. Nervenarzt. doi:10.1007/s00115-019-0749-8
Koch H, Garcia AJ, Ramirez JM. 2011. Network reconfiguration and neuronal plasticity in rhythm-generating networks. Integr Comp Biol 51:856–868. doi:10.1093/icb/icr099
Layer N, Müller P, Ayash M, Pfeiffer F, Saile M, Klopfer F, Iavarone S, Santuy A, Fallier-Becker
P, Hedrich UBS, Lerche H, Koch H, Wuttke T V. 2023. Axonopathy and altered synaptic development in early hippocampal epileptogenesis of Dravet syndrome. bioRxiv 2023.10.04.560735. doi:10.1101/2023.10.04.560735
Layer N, Sonnenberg L, Pardo González E, Benda J, Hedrich UBS, Lerche H, Koch H, Wuttke T V. 2021c. Dravet Variant SCN1AA1783V Impairs Interneuron Firing Predominantly by Altered Channel Activation. Front Cell Neurosci 15. doi:10.3389/fncel.2021.754530
Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YWA, Garcia AJ, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. 2012a. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci 15:793–802. doi:10.1038/nn.3078
Marcuccilli CJ, Tryba AK, Van Drongelen W, Koch H, Viemari JC, Peña-Ortega F, Doren EL, Pytel P, Chevalier M, Mrejeru A, Kohrman MH, Lasky RE, Lew SM, Frim DM, Ramirez JM. 2010. Neuronal bursting properties in focal and parafocal regions in pediatric neocortical epilepsy stratified by histology. J Clin Neurophysiol 27:387–397. doi:10.1097/WNP.0b013e3181fe06d8
Martell A, Dwyer J, Koch H, Zanella S, Kohrman M, Frim D, Ramirez JM, Van Drongelen W. 2010. N-Methyl-d-Aspartate-Induced oscillatory properties in neocortical pyramidal neurons from patients with epilepsy. J Clin Neurophysiol 27:398–405. doi:10.1097/WNP.0b013e3182007c7d
Moya-Díaz J, Bayonés L, Montenegro M, Cárdenas AM, Koch H, Doi A, Marengo FD. 2020. Ca2+-independent and voltage-dependent exocytosis in mouse chromaffin cells. Acta Physiol 228. doi:10.1111/apha.13417
Quintana Albert, Zanella S, Koch H, Kruse SE, Lee D, Ramirez JM, Palmiter RD. 2012. Fatal breathing dysfunction in a mouse model of Leigh syndrome. J Clin Invest 122:2359–2368. doi:10.1172/JCI62923
Ramirez JM, Koch H, Garcia AJ, Doi A, Zanella S. 2011. The role of spiking and bursting pacemakers in the neuronal control of breathing. J Biol Phys 37:241–261. doi:10.1007/s10867-011-9214-z
Shea SD, Koch H, Baleckaitis D, Ramirez JM, Margoliash D. 2010. Neuron-specific cholinergic modulation of a forebrain song control nucleus. J Neurophysiol 103:733–745. doi:10.1152/jn.00803.2009
Theiss S, Maetzler W, Deuschle C, Lerche H, Koch H, Dihné M. 2017. Dementia with Lewy bodies: Cerebrospinal fluid suppresses neuronal network activity. Neuroreport 28:1061–1065. doi:10.1097/WNR.0000000000000890
Van Drongelen W, Koch H, Elsen FP, Lee HC, Mrejeru A, Doren E, Marcuccilli CJ, Hereld M, Stevens RL, Ramirez JM. 2006. Role of persistent sodium current in bursting activity of mouse neocortical networks in vitro. J Neurophysiol 96:2564–2577. doi:10.1152/jn.00446.2006
Van Drongelen W, Koch H, Marcuccilli C, Peña F, Ramirez JM. 2003. Synchrony levels during evoked seizure-like bursts in mouse neocortical slices. J Neurophysiol 90:1571–1580. doi:10.1152/jn.00392.2003
Yang D, Qi G, Ort J, Witzig V, Bak A, Delev D, Koch H, Feldmeyer D. 2023. Modulation of Giant Depolarizing Potentials (GDPs) in Human Large Basket Cells by Norepinephrine and Acetylcholine. bioRxiv 2023.01.02.522475. doi:10.1101/2023.01.02.522475
Yuste R, Hawrylycz M, Aalling N, Aguilar-Valles A, Arendt D, Armañanzas R, Ascoli GA, Bielza C, Bokharaie V, Bergmann TB, Bystron I, Capogna M, Chang YJ, Clemens A, de Kock CPJ, DeFelipe J, Dos Santos SE, Dunville K, Feldmeyer D, Fiáth R, Fishell GJ, Foggetti A, Gao X, Ghaderi P, Goriounova NA, Güntürkün O, Hagihara K, Hall VJ, Helmstaedter M, Herculano-Houzel S, Hilscher MM, Hirase H, Hjerling-Leffler J, Hodge R, Huang J, Huda R, Khodosevich K, Kiehn O, Koch H, Kuebler ES, Kühnemund M, Larrañaga P, Lelieveldt B, Louth EL, Lui JH, Mansvelder HD, Marin O, Martinez-Trujillo J, Chameh HM, Mohapatra AN, Munguba H, Nedergaard M, Němec P, Ofer N, Pfisterer UG, Pontes S, Redmond W, Rossier J, Sanes JR, Scheuermann RH, Serrano-Saiz E, Staiger JF, Somogyi P, Tamás G, Tolias AS, Tosches MA, García MT, Wozny C, Wuttke T V., Liu Y, Yuan J, Zeng H, Lein E. 2021 Classification and nomenclature of neocortical cell types (Nature Neuroscience, (2020), 23, 12, (1456-1468), 10.1038/s41593-020-0685-8). Nat Neurosci. doi:10.1038/s41593-020-00779-0

