The Gründer Lab
The aim of the Gründer lab is to characterize at the functional and structural level ion channels of the DEG/ENaC gene family. We strive to identify fundamental principles and to produce high-quality data that stand the test of time.

Our research has a focus on ion channels. Ion channels are the basis of neuronal excitability and of communication between neurons. Mutations in ion channels can lead to diseases and malregulation of ion channels determines the clinical picture of many diseases. The analysis of ion channels in molecular detail contributes to a better understanding of their pathophysiological role and is the basis for educated pharmacotherapy.
Currently we focus on ion channels of the DEG/ENaC gene family. We are especially interested in 3 subfamilies, which contain closely related ion channels that are activated by 3 different extracellular ligands:
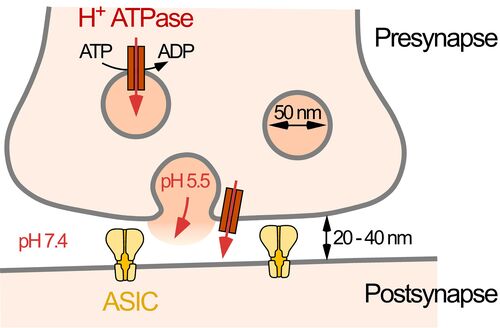
Acid-sensing ion channels (ASICs) are major receptors for extracellular protons; they are broadly expressed in neurons of the central and peripheral nervous system. ASICs contribute to synaptic transmission and sensory transduction. Because protons are also released during an ischemia, the activation of ASICs contributes to angina pectoris and cell death that accompanies ischemic stroke. In addition, ASICs contribute to axonal degeneration in an animal model for multiple sclerosis. Thus, ASICs are of great physiological and pathophysiological importance. A detailed understanding of their activation and regulation are, thus, of great relevance. Currently, we focus on the characterization of the ASIC1a proteome - ASIC1a and its auxiliary proteins.
Three relevant key publications of our group:
- Bartoi T, Augustinowski K, Polleichtner G, Gründer S, und Ulbrich MH (2014) Acid-sensing ion channel (ASIC) 1a/2a heteromers have a flexible 2:1/1:2 stoichimetry. Proc. Natl. Acad. Sci. USA, 111, 8281-8286
- Reimers C, Lee C-H, Kalbacher H, Tian Y, Hung C-H, Schmidt A, Prokop L, Kauferstein S, Mebs D, Cheng C-C and Gründer S (2017) Identification of a cono-RFamide from the venom of Conus textile that targets ASIC3 and enhances muscle pain. Proc. Natl. Acad. Sci. USA 114(17), E3507-E3515
- Fischer L, Schmidt A, Dopychai A, Joussen S, Joeres N, Oslender-Bujotzek A, Schmalzing G, and Gründer S (2023) Physiologically relevant acid-sensing ion channel (ASIC) 2a/3 heteromers have a 1:2 stoichiometry. Commun. Biol., 6(1): 701
The bile acid-sensitive ion channel (BASIC) is a sparsely characterized ion channel that is expressed in bile ducts and that is directly activated by bile salts. In bile ducts and the small intestine, BASIC is potentially involved in Na+ transport. Therefore, BASIC might have a potential impact on diarrhea and cholestasis. In addition, BASIC is present in brain, where its function is not understood yet. Currently, we investigate the role of BASIC in the liver, the pancreas, and the intestine.

Three relevant key publications of our group:
- Wiemuth D, Sahin H, Falkenburger BH, Lefèvre C, Wasmuth HE und Gründer S (2012) BASIC - a bile acid-sensitive ion channel highly expressed in bile ducts.FASEB J., 26, 4122-4130
- Schmidt A, Löhrer D, Alsop RJ, Lenzig P, Oslender-Bujotzek A, Wirtz M, Rheinstädter MC, Gründer S, and Wiemuth D (2016) A cytosolic amphiphilic alpha helix controls the activity of the Bile Acid Sensitive Ion Channel BASIC.J. Biol. Chem. 291(47): 24551-24565
- Wiegreffe S, Löhrer D, Wirtz M, and Wiemuth D (2021) The bile acid-sensitive ion channel (BASIC) mediates bile acid-dependent currents in bile duct epithelial cells. Pflügers Arch. 473(12): 1841–1850
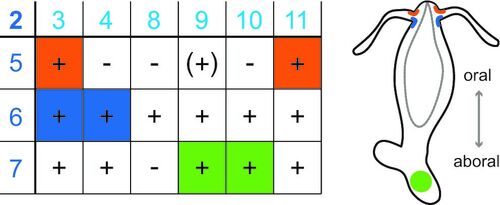
Peptide-gated ion channels are only sparsely investigated. Biological dogma holds that peptides only gate G-protein-coupled receptors. We could isolate a group of peptide-gated ion channels, the Hydra Na+ channels (HyNaCs), from the freshwater polyp Hydra magnipapillata, showing that neuropeptides were already used for fast synaptic transmission in simple nervous systems. Neuropeptides are common also in humans and ASICs are modulated by neuropeptides. We investigate HyNaCs as a model channel to better understand neurotransmission in a simple nervous system and to identify conserved features in the closely related ASICs and BASIC.
Three relevant key publications of our group:
- Golubovic A, Kuhn A, Williamson M, Kalbacher H, Holstein TW, Grimmelikhuijzen CJ und Gründer S (2007) A peptide-gated ion channel from the freshwater polyp Hydra.J. Biol. Chem. 282: 35098-35103
- Assmann M, Kuhn A, Dürrnagel S, Holstein TW and Gründer S (2014) The comprehensive analysis of DEG/ENaC subunits in Hydra reveals a large variety of peptide-gated channels, potentially involved in neuromuscular transmission. BMC Biology 12: 84
- Schmidt A, Bauknecht P, Williams EA, Augustinowski K, Gründer S*, and Jékely G* (2018) Dual signaling of Wamide myoinhibitory peptides through a peptide-gated channel and a GPCR in Platynereis. FASEB J. 32: 5338-5349
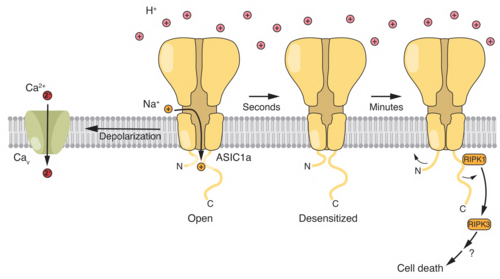
In addition, we investigate the role of ion channels and acid-base transporters in brain tumors, such as glioblastoma and medulloblastoma.
Three relevant key publications of our group:
- Tian Y, Bresenitz P, Reska A, El Moussaoui L, Beier C and Gründer S (2017) Glioblastoma cancer stem cell lines express functional acid sensing ion channels ASIC1a and ASIC3. Sci. Rep. 7: 13674
- Clusmann J, Franco KC, Suárez DAC, Katona I, Minguez MG, Boersch N, Pissas KP, Vanek J, Tian Y, and Gründer S (2022) Acidosis induces RIPK1-dependent death of glioblastoma stem cells via acid-sensing ion channel 1a. Cell Death Dis., 13(8): 702
- Cortés Franco KD, Brakmann IC, Feoktistova M, Panayotova-Dimitrova D, Gründer S, and Tian, Y (2023) Aggressive migration in acidic pH of a glioblastoma cancer stem cell line in vitro is independent of ASIC and KCa3.1 ion channels, but involves phosphoinositide 3-kinase. Pflügers Arch., 475(3): 405-416



